Building Digital Infrastructure
for a Collaborative, Innovative Life Sciences Ecosystem
Transforming Pharma with Collaborative Digital Infrastructure and Innovation
Accelerating Drug Approval and Ensuring Patient Safety with
Digital Technology Making Quality Medicines Accessible and Reducing Patient Costs
Pharmaceutical Companies and CROs
Use Our Products and Services
National Clinical Trial Institutions
Partnered with Us
SMO
Have Partnered with Us
Cross-Disciplinary Pharma and IT Experts
Ensuring Innovation and Expertise
Software Products and Digital Services
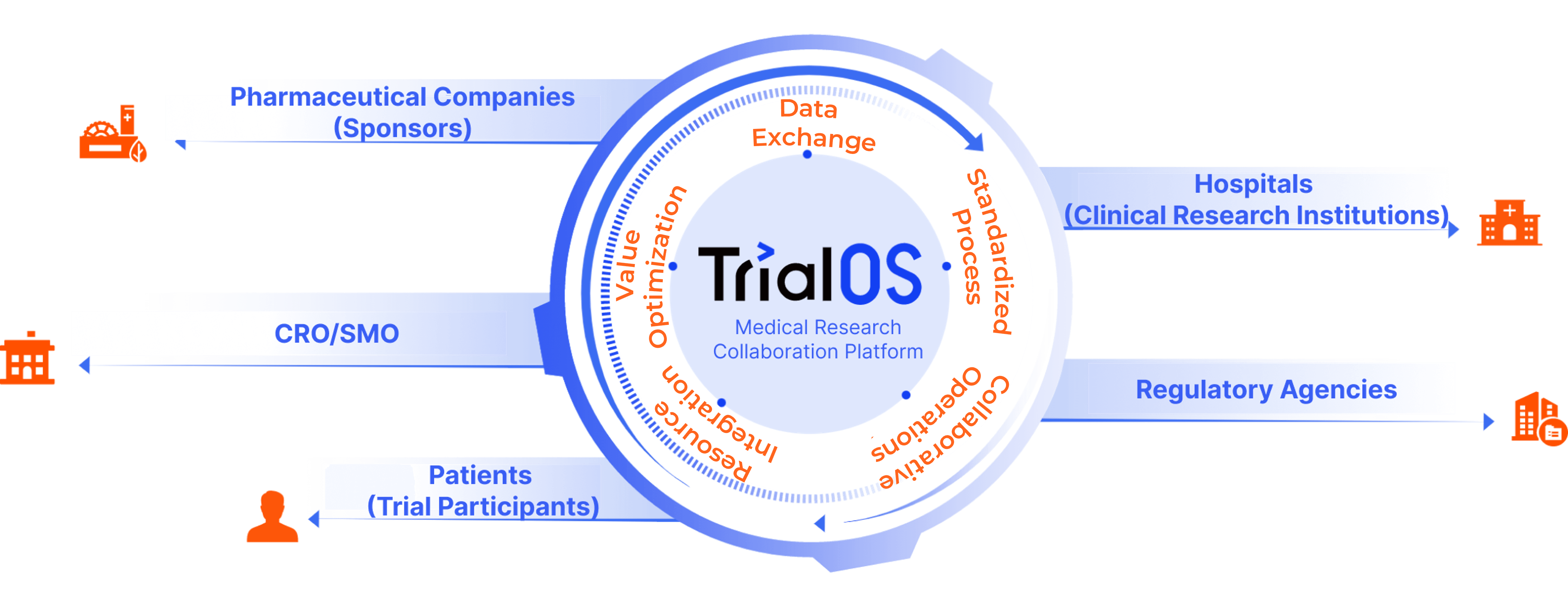
Unified login portal linking industry stakeholders, standardizing data flow, breaking data silos, and boosting clinical research efficiency.
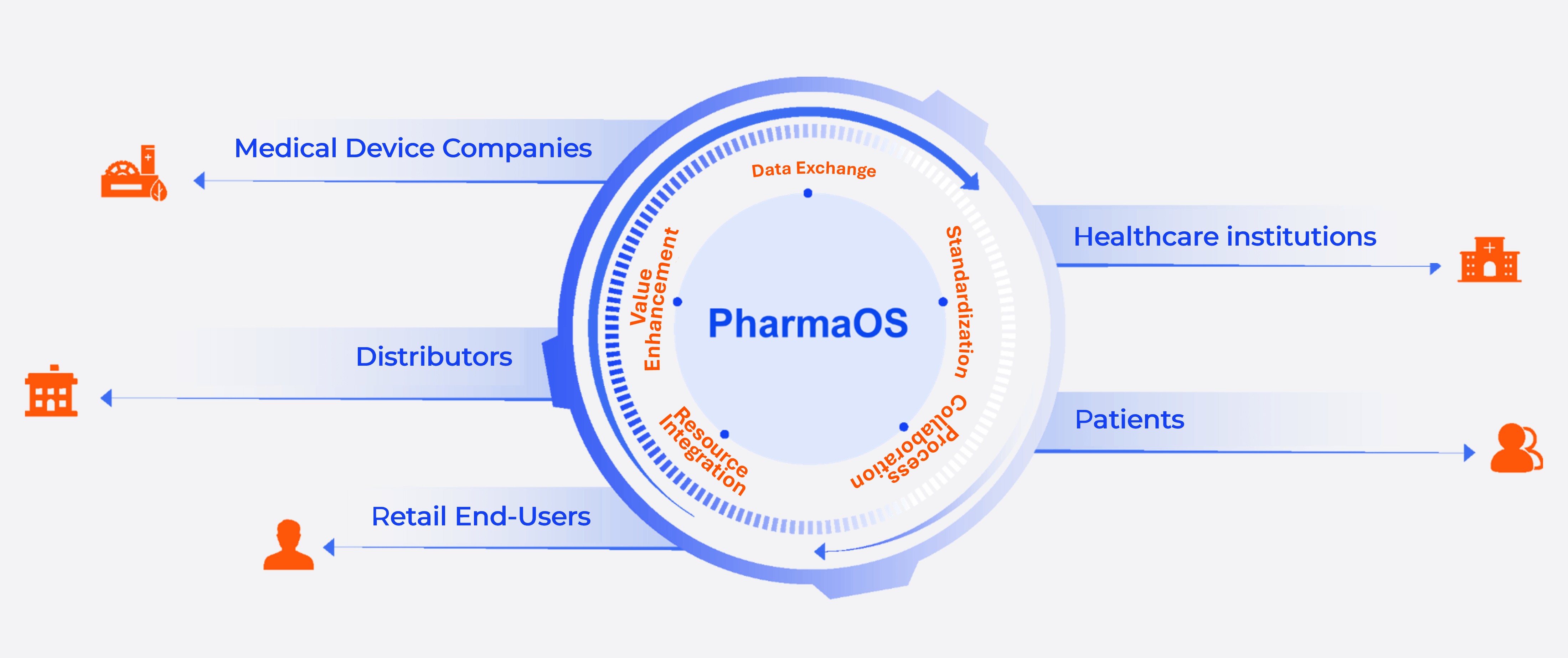
Integrate the omni-channel marketing system and marketing data middle platform to break data silos, provide end-to-end marketing digital solutions based on data, deeply cultivate target customers in multiple dimensions, refine sales management, make accurate decisions, and improve business performance.

TrialOS offers a unified login portal connecting pharmaceutical companies, hospitals, third-party providers, patients, and regulatory agencies. It standardizes processes, enables data flow, breaks data silos, fosters collaboration, integrates resources, and boosts overall efficiency in clinical research.

Integrating an omnichannel marketing system and centralized marketing data platform, PharmaOS breaks down data silos to offer data-driven, end-to-end digital marketing solutions. This enables comprehensive targeting of key customers, refined sales management, precise decision-making, and improved business performance.







Scan to Follow Us on LinkedIn